Alternative Lengthening of Telomeres
Telomeres are specialized structures at the ends of the linear chromosomes. Their maintenance is essential for the unlimited proliferation of cells due to the 3'-end erosion, a process intrinsic to the replication of linear chromosomes. Progressive telomere shortening in somatic cells can lead to the induction of senescence or apoptosis, thus acting as a barrier to unlimited proliferation and tumorigenesis. Accordingly, inhibition of telomere length maintenance is considered an important goal in tumor therapy. Most cancer cells reactivate the reverse transcriptase telomerase to maintain their telomeres. However, 10-15 % of cancer cells use the repair-based ALT pathway instead. The ALT mechanism is restricted to cancer cells and immortalized cell lines, and treatment of telomerase-positive tumors with telomerase inhibitors could select for those cells within the cancer cell population which are capable to survive via the ALT mechanism. Therefore, understanding ALT is crucial for (i) the identification of tumors in which it is active, (ii) the development of prognostic markers, and (iii) the targeting of this pathway with novel anti-cancer therapies.
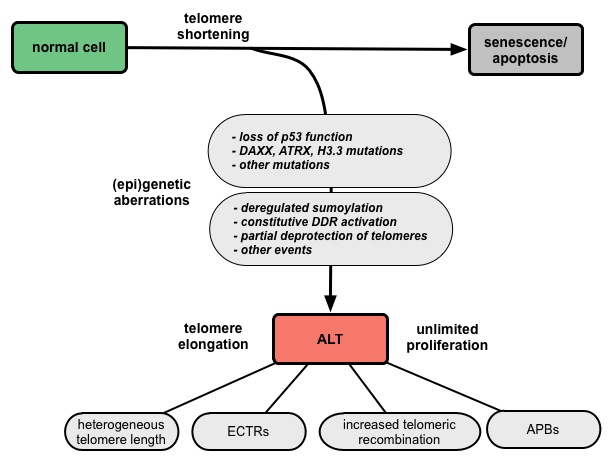
ALT-positive cells are characterized by an extreme heterogeneity of telomere repeat length, an elevated level of telomere sister chromatid exchanges (T-SCEs), extrachromosomal telomere repeats (ECTRs), and the presence of ALT-associated PML (promyelocytic leukemia) bodies (APBs) (Fig. 1). Since APBs contain proteins that are known to function in DNA repair and recombination processes it is thought that the telomere elongation takes place in these nuclear structures. Several mutations and deregulations involved in the ALT pathway were discovered, although the molecular mechanism remains elusive (Fig. 2).
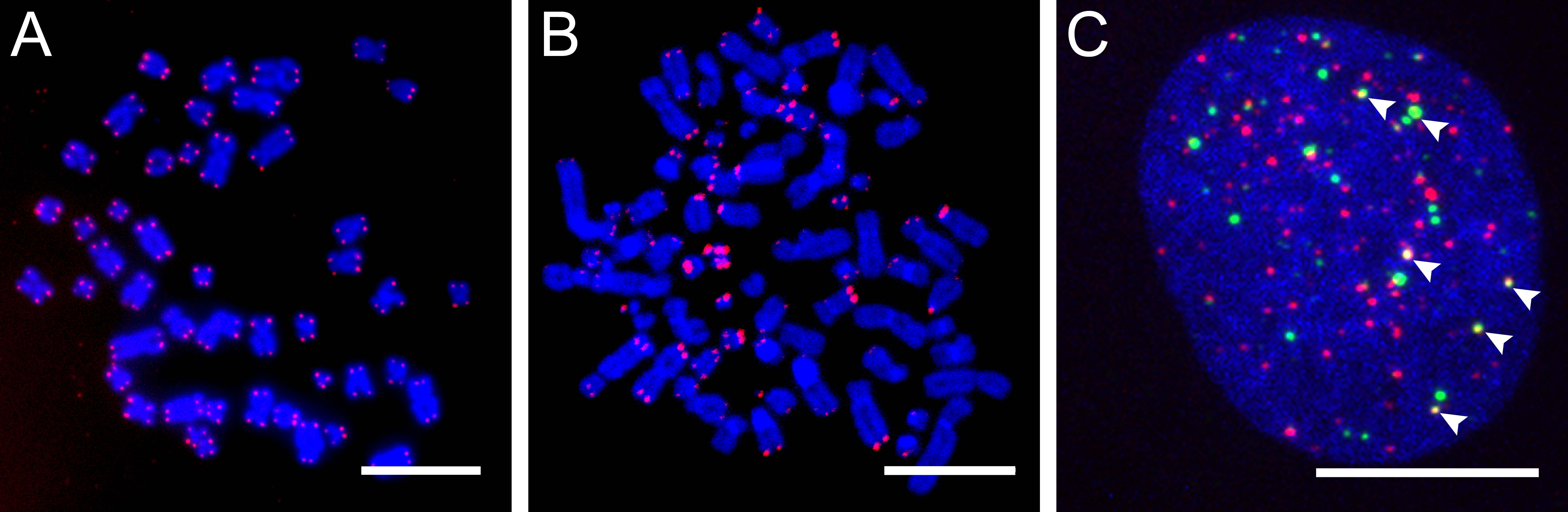 |
Figure 1. Characteristic features of ALT-positive cells. |
Figure 2. Model for the development of ALT via multiple deregulation steps. |
 |
We have presented results on the mobility of telomeres and PML bodies as well as their interaction in human U2OS osteosarcoma cells, in which the ALT pathway is active (Jegou et al., 2009). An U2OS cell line was investigated that has lac operator repeats stably integrated adjacent to the telomeres of three different chromosomes. Using a fusion protein of the lac repressor LacI, which binds with high affinity to the lacO repeats, and a GFP binding protein (GBP), GFP-tagged proteins can be recruited to these regions. With this system we have investigated the role of PML bodies in the ALT pathway regarding their composition, assembly and functionality (Chung et al., 2011). Using high-resolution 4Pi microscopy, we could show that PML forms a spherical shell around the telomere (Lang et al., 2010). Furthermore, we systematically identified proteins involved in the maintenance of telomeres in an ALT-positive cancer cell line. The proteins involved were identified in an high-content confocal RNA interference screen using APBs as readout (Osterwald et al., 2012).
In our current projects, we investigate the potential role of the telomeric repeat containing RNA (TERRA) in the ALT pathway. Using the high-content confocal screening platform, the mechanism by which APBs induce telomere lengthening in ALT-positive tumor cells is studied.
References
- Chung, I., Osterwald, S., Deeg, K., and Rippe, K. (2012) PML body meets telomere: the beginning of an ALTernate ending? (Review) Nucleus, 3(3), 263-275.
- Osterwald, S., Woerz, S., Reymann, J., Sieckmann, F., Rohr, K., Erfle, H., Rippe, K. (2012) A three-dimensional colocalization RNA interference screening platform to elucidate the alternative lengthening of telomeres pathway. Biotechnol. J., 7(1), 103-116.
- Chung, I., Leonhardt, H., and Rippe, K. (2011) De novo assembly of a PML nuclear subcompartment occurs via multiple pathways and induces telomere elongation. Journal of Cell Science 124, 3603-3618.
- Lang, M., Jegou, T., Chung, I., Richter, K., Münch, S., Udvarhelyi, A., Cremer, C., Hemmerich, P., Engelhardt, J., Hell, S., and Rippe, K. (2010) Three-dimensional organization of promyelocytic leukemia nuclear bodies. Journal of Cell Science 123, 392-400.
- Jegou, T., Chung, I., Heuvelmann, G., Wachsmuth, M., Görisch, S.M., Greulich-Bode, K., Boukamp, P., Lichter, P., and Rippe, K. (2008) Dynamics of telomeres and PML nuclear bodies in a telomerase negative human cell line. Molecular Biology of the Cell 20, 2070-2082.
People working on this project
Applied methods & techniques
Fluorescence Imaging
Single Cell Manipulations
Fluctuation Microscopy
Fluctuation Microscopy
